Reports
Novel Insulin-Independent Mechanism of Action Hits Triple Targets in Type 2 Diabetes
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
MEDICAL FRONTIERS - 4th World Congress on Controversies to Consensus in Diabetes, Obesity and Hypertension (CODHy)
Barcelona, Spain / November 8-11, 2012
Barcelona - Management of type 2 diabetes mellitus (T2DM) implies more than achieving good glycemic control as most patients have comorbid conditions including high blood pressure (BP) and dyslipidemia. Most are overweight or obese as well. Careful treatment of all comorbid conditions can be expected to improve micro and macrovascular complications but some antihyperglycemic agents, especially insulin and the sulphonylureas, are associated with weight gain and hypoglycemia. Sodium-glucose co-transporter 2 (SGLT2) inhibitors are a new class of antihyperglycemic agents that is showing considerable promise for the treatment of T2DM. Their most important property is the ability to lower blood glucose through a completely insulin-independent mechanism of action, virtually obviating the risk of hypoglycemia. By promoting glycosuria, the SGLT2 inhibitors induce weight loss and being sodium-dependent, they reduce BP as well. Studies to date indicate that the SGLT2 inhibitors may be used as add-on therapy to virtually any antihyperglycemic regimen with the expectation that their addition will further reduce HbA1c, weight and BP simultaneously.
Chief Medical Editor: Dr. Léna Coïc, Montréal, Quebec
Achieving target HbA1c levels of <7% as recommended for most patients with type 2 diabetes mellitus (T2DM) is only one challenge in the management of this increasingly common chronic condition. Approximately three-quarters of T2DM patients are also hypertensive with many being overweight or obese, and most have some form of dyslipidemia. Not inconsequentially, as diabetes progresses, the presence of chronic kidney disease (CKD) complicates disease management with kidney failure becoming increasingly likely.
Treatment Challenges
As discussed by Prof. Luc Van Gaal, Professor of Medicine, Antwerp University Hospital, Belgium, part of the therapeutic challenge in T2DM is inherent to the antihyperglycemic agents themselves. Insulin is associated with weight gain and hypoglycemia but so, too, are many of the oral antihyperglycemic agents including the sulphonylureas (SU) and the thiazolidinediones. If patients have CKD, with an estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2, “the risk of hypoglycemia is even more severe and there is not one single treatment approach that is optimal for these patients,” Prof. Van Gaal told delegates.
Repeated episodes of hypoglycemia have been associated with a number of adverse outcomes including weight gain. “Patients with severe hypoglycemic [episodes] often adopt a defensive snacking behaviour to either avoid hypoglycemia or to compensate for it,” he explained, a cause for more weight gain. In T2DM, weight gain is typically the accumulation of intra-abdominal or visceral fat, clearly linked to an excess risk of cardiovascular (CV) events.
Intensive Intervention Strategy: The STENO-2 Evidence
To offset the amplified CV risk in patients with T2DM, physicians need to approach the disease and its complications on multiple fronts. This multi-factorial approach was best exemplified by results from the STENO-2 study. In high-risk patients with T2DM, STENO-2 investigators showed that an intensive intervention strategy with multiple drug combinations as well as behaviour modification led to sustained clinical benefits with respect to vascular complications and all-cause mortality as well as CV death. The benefits of this comprehensive approach were already evident as early as 3 years into the study (N Engl J Med 2008:358:580-91) (Table 1).
Table 1.
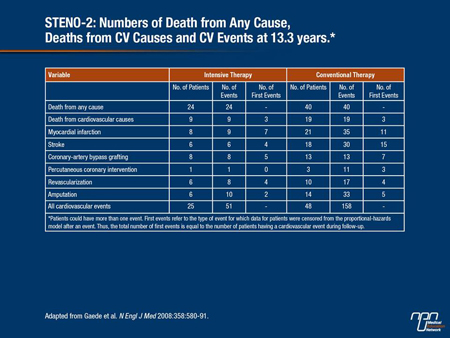
“If you treat your patients including those with compromised kidney function with a multi-factorial approach, they will have better long-term outcomes,” Prof. Van Gaal affirmed.
Overcoming Clinical Inertia
Given that most physicians are aware that current antihyperglycemic agents are less than ideal, there is a certain clinical inertia when it comes to initiating or escalating therapy in T2DM. In one study cited by co-chair, Dr. Manual Puig-Domingo, Hospital Universitari Germans, Barcelona, Spain, Canadian investigators estimated how many patients with T2DM managed in the primary care setting achieved triple targets of a blood pressure (BP) ≤130/80 mm Hg, an HbA1c ≤7% and a LDL-C ≤2.5 mmol/L (Int J Clin Practice 2012;
66:457-64).
Management improved over time in response to the introduction of a quality enhancement research initiative. In more than 3000 patients followed over 12 months, while about 60%, 57% and 75% of patients reached BP, HbA1c and LDL-C targets, respectively, only 26% achieved all 3 targets. Other countries have similar proportions of patients with T2DM achieving individual targets or all 3.
Physician and patient factors both contribute to “clinical inertia,” as Dr. Puig-Domingo noted, but the end result is that many patients are not started on insulin until it is too late to prevent complications from the disease. One strategy to help overcome clinical inertia, Dr. Puig-Domingo suggested, is to stop using sequential treatments following failure of metformin, introducing insulin at a much earlier stage to improve glycemic control. Another would be to introduce more effective combinations of antihyperglycemic agents as soon as glycemic control becomes inadequate.
The Kidney and Regulation of Glucose Metabolism
In an effort to improve therapies for better glucose control, the kidney has become a potential therapeutic target. “The kidney is an important organ in the regulation of glucose metabolism in that it filters and reabsorbs circulating glucose on a continuous basis,” stated Dr. John Wilding, Head, Obesity and Endocrinology, University of Liverpool, UK. Under normal conditions, the kidneys are able to filter and reabsorb up to 180 g of glucose over a 24-hour period. To do this, the kidney relies on 2 sodium-dependent glucose transporters, the sodium-glucose co-transporter 2 (SGLT2), which is found only in the kidney and reabsorbs approximately 90% of renal glucose, and the SGLT1 transporter, which “mops up” the remaining 10%.
“When patients develop T2DM, you overcome the capacity of the system to reabsorb glucose so at around 10 to 11 mmol/L of plasma glucose, we start to see glucose in the urine,” Dr. Wilding noted, “and the amount of glucose in the urine will increase proportionally to the blood glucose because it has overcome the capacity of this reabsorptive mechanism,” glycosuria serves to reduce blood glucose levels.
Benefits of SGLT2 Inhibition
SGLT2 inhibitors favourably affect common comorbid features in T2DM, notably weight gain and hypertension. By increasing the excretion of glucose into the urine, approximately 100 g of glucose will be lost in the urine every day. This amount of glucose translates into about 200 to 300 calories a day—and that could potentiate weight loss, as Dr. Wilding observed. The SGLT2 transporters are also sodium-dependent, so as glucose is excreted into the urine, so, too, is sodium, thereby reducing BP. And because the SGLT2 inhibitors work entirely independently of insulin secretion or action, they are much less likely to lead to hypoglycemia.
“With these SGLT2 inhibitors, we might be able to target 3 of the main problems in T2DM, i.e. hyperglycemia, increased body weight and hypertension, with one therapy,” Dr. Wilding emphasized (Figure 1). “Hypoglycemia is another big problem in T2DM and it’s very important to try and avoid it when treating patients.”
Figure 1.
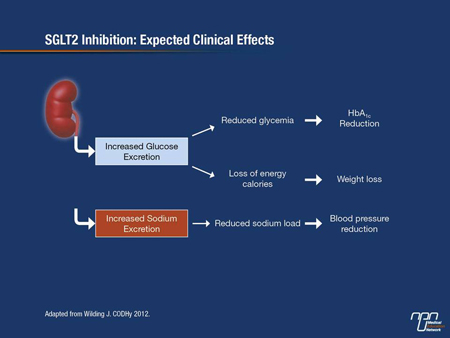
Clinical Trial Data
Several SGLT2 inhibitors are in phase III clinical trial programs. As Dr. Jochen Seufert, University Hospital Freiburg, Germany, reminded delegates, all current antihyperglycemic agents are dependent on insulin secretion or insulin action for glycemic control. “SGLT2 inhibition is completely insulin-independent so this mechanism of action may be additive to any other treatment paradigm,” he noted. Results from SGLT2 inhibitors in clinical trials so far indicate that they achieve good glycemic control as monotherapy or as add-on therapy to either metformin or on top of a dual antihyperglycemic regimen.
They are also all associated with weight loss, an expected result due to their mechanism of action. Because they also lead to sodium excretion, clinicians can expect to see moderate reductions in BP as well.
As Dr. Seufert noted, the increased glycosuria with SGLT2 inhibition increases the risk of genital bacteria and mycotic infections in both men and women. These do not appear to be problematic as patients respond to standard therapies and these do not tend to recur.
When the SGLT2 inhibitor canaglifozin (100 mg and 300 mg) was used as monotherapy on top of diet and exercise, Stenlöf et al. reported that the mean HbA1c reduction from baseline at 26 weeks was 0.77% for the 100 mg dose, 1.03% for the 300 mg dose compared with 0.14% for placebo (P<0.001 for both doses). Approximately 44% and 62% of patients on the 100 mg and 300 mg dose, respectively, achieved an HbA1c <7% compared with only 20% on placebo.
Dr. Seufert reported clinical trial results of dapagliflozin 10 mg added to metformin. The STGL2 inhibitor further reduced HbA1c levels by approximately 1.98% with a reduction in body weight of approximately 2.8 kg over 24 week.
At 52 weeks, HbA1c reductions with canagliflozin 100 mg added to background metformin demonstrated non-inferiority to the SU glimepiride and the reductions were statistically significant with the 300 mg dose.
In phase III trials, empagliflozin has been shown to reduce HbA1c in a dose-dependent manner, with additional reductions in weight relative to metformin—“so in addition to glucose-lowering, you get consistent reductions in body weight with SGLT2 inhibition,” Dr. Seufert emphasized.
Wilding et al. also reported that canagliflozin 100 and 300 mg also reduced HbA1c at 26 weeks by 0.85% and 1.06%, respectively, when added to metformin plus a SU. The mean reduction in body weight at 26 weeks for the 100 mg and 300 mg dose was 1.9 kg and 2.5 kg, respectively, compared with 0.8 kg for the placebo arm.
As Dr. Seufert observed, HbA1c levels predictably begin to rise after about 1 year of treatment with any SU, an effect that was seen in this trial as well. At study end point, a significant reduction in body weight was reported for canagliflozin 300 mg (-4.7 kg) vs. a weight gain of approximately 0.7 kg for the SU (P<0.001).
The DDP4 inhibitor sitagliptin is considered weight neutral. Investigators also showed a larger reduction in HbA1c when empagliflozin was added to metformin than when the DDP4 inhibitor sitagliptin was added to the same background therapy.
For canagliflozin 300 mg, results showed a mean reduction in HbA1c of 1.03% for compared to 0.66% with sitagliptin at 52 weeks.
When the SGLT2 inhibitor dapagliflozin was added to insulin in patients with poorly controlled HbA1c (baseline mean 8.5%), greater reductions in mean HbA1c were reported over 104 weeks with the 5 and 10 mg
doses at 0.64 to 0.82% respectively, compared with a 0.43% reduction for placebo. Initial weight loss in the dapagliflozin groups was maintained over 104 weeks as well, whereas weight continued to rise in placebo patients. There was also no increase in hypoglycemic episodes when it was added to insulin.
Nauck et al. also compared the long-term safety and efficacy of dapagliflozin to the insulin secretagogue glipizide added to background metformin in patients with inadequately controlled T2DM. The mean change in HbA1c of -0.52% at 52 weeks was identical for both agents.
However, at week 104, investigators noted that the reduction in HbA1c seen at 52 weeks with the SGLT2 inhibitor was sustained whereas glipizide’s effect on HbA1c was slightly attenuated by study end point. The mean difference in weight at study end point was 5.06 kg in favour of the SGLT2 inhibitor. Importantly, the proportion of patients with hygoglycemic episodes at study end point was also 10-fold lower with dapagliflozin (4.2%) than with glipizide (45.8%).
Average body weight reductions reached 3.0 to 3.5 kg in all canagliflozin studies evaluated as add-on therapy. Notably, all studies as add-on therapy, reported an average reduction in systolic BP (SBP) of approximately 5 mm Hg.
For example, another study in which canagliflozin (100 and 300 mg) was added to metformin and pioglitazone showed that both doses of the SGLT2 inhibitor reduced HbA1c by 0.89% and 1.03% respectively at the end of 26 weeks vs. 0.26% with placebo (P<0.001). Greater reductions in body weight of 2.6 and 3.7 kg at study end point were reported on both canagliflozin doses as well as reductions in systolic BP of approximately 5 mm Hg for both doses.
“If you look at the big antihypertensive studies with the ACE inhibitors or the ARBs, a 5 mm Hg reduction on average is what you get with antihypertensive drugs as well, so with these agents, reduction in BP is clinically very relevant,” Dr. Seufert remarked.
Importantly, canagliflozin proved effective in patients with moderate renal impairment (eGRF of >30 and <50 mL/min/1.73 m2). Yale et al. reported that the 100 mg and 300 mg doses reduced HbA1c by a mean of 0.33% and 0.44% from baseline, respectively, compared to 0.03% for placebo controls. Reductions in both HbA1c and body weight with SGLT2 inhibition are more attenuated in patients with modest renal failure and SGLT2 inhibitors would not be suitable for use in patients with severe renal impairment, as Dr. Seufert cautioned.
Nevertheless, SGLT2 inhibition may represent a potential new therapeutic option for patients with T2DM and moderate renal impairment, speakers here agreed.
Summary
With HbA1c levels in many patients with T2DM not adequately controlled, better antihyperglycemic agents are needed. The novel SGLT2 inhibitors represent a new therapeutic class in diabetes management in that they effectively lower HbA1c when added to any other regimen and also reduce weight and BP. This suggests that physicians may be able to target 3 important features of T2DM with a single therapy, which represents a new therapeutic paradigm for improved diabetes control.