Reports
Immunization of School Pupils: What Ontario Physicians Should Know
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
MEDI-NEWS - Based on Government of Ontario Immunization of School Pupils Act
July 2014
The Ontario government has recently modified immunization requirements for school-age children, mandating receipt of the meningococcal, pertussis and varicella vaccines for school entry. The clinical implications of these new requirements need to be carefully considered by Ontario physicians as there is need to take a second look at the new requirements and the epidemiological reality of invasive meningococcal disease (IMD) in much of Canada, including Ontario, today. IMD is a rare but potentially catastrophic disease that can prove rapidly fatal and from which at least at least one-fifth of patients suffer significant sequelae. Until recently, no vaccine was available that offered any protection against what is now the most common serogroup causing IMD in Canada. Late in 2013, a multicomponent meningococcal B vaccine (4CMenB or Bexsero) was approved for use in individuals from the age of 2 months through 17 years where the risk of serogroup B disease is highest.
Chief Medical Editor: Dr. Léna Coïc, Montréal, Quebec
If Ontario physicians aren’t already doing so, they are soon likely to be providing more school-age vaccinations than usual as parents scramble to comply with new immunization requirements before their children head back to school in September.
As of July 2014, the Ontario government will require proof of immunization against meningococcal disease, pertussis and varicella in order for children to attend school in Ontario. As designated in the new schedule, the legislation states that these 3 immunizations will be enforced (see Table 1). The same legislation also updated the number of doses and intervals between doses for tetanus, diphtheria, poliomyelitis, measles, mumps and rubella; a second dose of the mumps vaccine is now required as well.
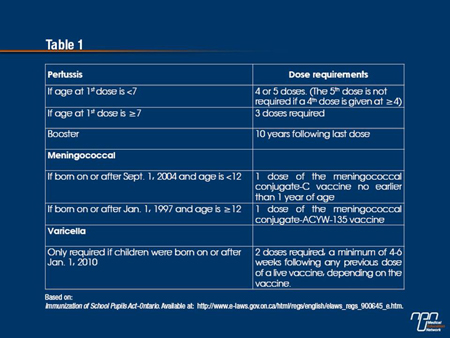
Children who do not have the required immunization records or valid exemptions may be temporarily suspended from school by order of the local medical officer of health.
“Many harmful diseases have been nearly eradicated in Ontario because of publicly-funded vaccines,” Deb Matthews, then Minister of Health and Long-Term Care said in a press release. “That’s why our government is updating the requirements for school-aged children to be protected from vaccine-preventable diseases.”
Open for Discussion
Just how well children will be protected against meningococcal disease is, however, open for discussion. In Canada, 5 serogroups are responsible for invasive meningococcal disease (IMD), of which serogroup B is now the most prevalent, especially among infants under 1 year of age (80%); children 1 to 4 years of age (67%) and adolescents 15 to 19 years of age (62%) (Canada Communicable Disease Report 2013;39:ACS-1:1-40). In March 2014, guidelines prepared by the Meningococcal B Pilot Project Task Group and endorsed by the National Advisory Committee on Immunization (NACI) stated that physicians may now consider using the new multi-component vaccine against serogroup B Neisseria meningitidis (4CMenB or Bexsero) in infants 2 months of age and older to prevent IMD caused by serogroup B bacterium.
The recommendations also state that the same vaccine should be given to patients 2 months of age and older at high risk for—or who have been in close contact with—any person who developed IMD caused by serogroup B. The rationale behind these new recommendations is based on what might be thought of as the epidemiological imperative driving IMD in much of Canada today. Following widespread introduction of the meningococcal C conjugate vaccine, IMD caused by serogroup C has almost disappeared. In point of fact, the incidence of meningococcal B in Ontario has always been higher than all other serogroups even prior to the introduction of public programs against meningococcal C and against meningococcal serogroups ACW and Y, according to Kinlin et al (Vaccine 2009;27:1735-40).
In 2011, for example, 62% of IMD in the country was caused by serogroup B compared with only 2% caused by serogroup C. Between 2007 and 2011, an average of 192 cases of IMD a year was reported in Canada—an average of 111 of them due to serogroup B. An analysis of IMD in Ontario between 2000 and 2010 (BMC Infect Dis 2012;12:202) found that serogroup B was the most common cause of IMD in the province during the study interval and that the incidence was highest in infants, mostly those under 6 months of age. In this particular series, the case-fatality rate was 10.7% overall. IMD is also associated with significant morbidity including hearing and limb loss as well as cognitive deficits, according to the Task Group report.
Point of View
Dr. Paul Roumeliotis is the CEO and Medical Officer of Health of the Eastern Ontario Health Unit, Cornwall, Ontario. “I’m also a pediatrician so I know what meningococcal disease is and the devastation it can result in, no question about it,” he said in an interview.
Dr. Roumeliotis also believes that the 4CMenB vaccine “adds to the protection we already have against meningococcal disease.” Clinical trial evidence strongly supports the protective potential of the 4CMenB vaccine. No fewer than 10 clinical trials involving roughly 5800 subjects—approximately 4000 of whom were infants between 2 and 24 months of age—evaluated the immunogenicity of the new serogroup B vaccine, immune responses to each vaccine antigen being assessed independently using a human complement serum bactericidal assay titre against reference strains.
As summarized by the Task Group, the 4CMenB vaccine was found to be immunogenic after at least 2 doses in infants 12 months of age and younger (See Table 2). An anamnestic response to a booster dose, given at 12 months of age, was also evident and similar immune responses were observed when the vaccine was given together with other routine childhood vaccines as when it was given separately. Results in older children between 12 and 24 months of age found the 4CMenB vaccine was again immunogenic after 2 doses against vaccine reference strains.
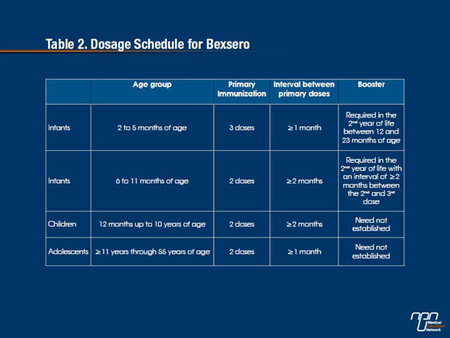
Trials of the serogroup B vaccine in adolescents and adults indicate that most adolescents achieved protective antibody responses against each of the 3 reference strains after 2 to 3 doses, as did adults after receiving 2 doses.
In children 2 years of age and under, fever has been more common after the first and second dose of the vaccine than after the third dose but it consistently occurs mostly within the first 6 hours of vaccine receipt.
Importantly, acetaminophen has been found to significantly lower the incidence of febrile events without affecting the immunogenicity of either the 4CMenB vaccine or any other routine vaccines given concomitantly with it.
Given this, the Task Group suggests that routine prophylactic administration of acetaminophen may be an appropriate strategy to reduce fever in infants receiving the 4CMenB vaccine.
Matter of Timing
From Dr. Roumeliotis’ perspective, the apparent disconnect between the Ontario government’s new legislation mandating meningococcal vaccines against all serogroups except serogroup B disease is probably just a matter of timing. “I can tell you, all new vaccines are looked at,” said Dr. Roumeliotis. “When this new legislation came out it was in October of last year and the [4CMenB] vaccine did not have NOC (Notice of Compliance) until December of 2013. So it was difficult to come up with a recommendation for a vaccine that wasn’t approved at the time. But I think that we will bring this matter back to the government, probably in the fall. It’s just a matter of time that these things will be looked at again.”
In the meantime, at a practical level, physicians need to be talking to their patients and discussing what’s available and what their options are. “After all, when the human papilloma virus (HPV) vaccine came out, we talked about it for a while and eventually it was publicly funded,” Dr. Roumeliotis noted. The fact that the new Ontario legislation does not address the need for vaccination against serogroup B IMD doesn’t mean that these discussions won’t happen in the future, he added; rather, it will just take more dialogue and time.
Some insurance companies likely cover the 4CMenB vaccine and it is likely that certain families can afford it even if it is not covered, he added. On the other hand, “one of the things we look at in public health is equity and at the moment our approach to the use of this vaccine is not equitable which is unfortunate,” Dr. Roumeliotis said, adding:
“It takes a while to get advocacy and change, but I will bring this [matter] to the attention of the chief medical officer of health [when appointed],” he said. “So it’s a matter of bringing this issue back [to the government] when a new government’s in place.”
4CMenB in Quebec
Quebec has the highest incidence of IMD caused by strains of serogroup B although there are regional differences. Because certain health regions appear to be disproportionately affected by serogroup B strains, the Ministry of Health and Social Services of Quebec recently asked the appropriate authorities to evaluate the potential of offering the 4CMenB vaccine to at-risk individuals living in these areas. Following deliberation of the proposal, members of the committee unanimously recommended individuals 20 years of age and younger who reside in an area most affected by serogroup B strains receive vaccination against serogroup B in an effort to better control the endemic situation in this region. As of June 19, 2014, the first wave of the Quebec regional intervention was completed and more than 81% of the targeted population (45,638 indivdiduals) have now received 1 dose of Bexsero.
For other areas in Quebec where the incidence rates of serogroup B strains are relatively high as well, the same authorities recommended enhanced surveillance of IMD epidemiology.
MN