Reports
New Protease Inhibitor Data Support Large Leap in Hepatitis C Virus Control
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
MEDICAL FRONTIERS - 48th Annual Meeting of the European Association for the Study of the Liver
Amsterdam, The Netherlands / April 24-28, 2013
Amsterdam - In the rapidly evolving field of hepatitis C virus (HCV), protease inhibitors (PIs) are poised to provide additional incremental increases to sustained virologic remission (SVR) rates in difficult cases. Since their introduction, PIs have substantially improved the likelihood of SVR in resistant genotypes, but new data presented at the European Association for the Study of the Liver (EASL) show substantial promise from simplified regimens administered over a shorter period. These regimens not only promise to address the problem of adherence but may reduce exposure to adverse events. Early clinical data with novel drugs coming forward in HCV treatment suggest that the first combinations to provide SVR without exposure to pegylated interferon and ribavirin may be on the horizon. By increasing the likelihood of SVR, PIs are substantially lowering the risk of catastrophic complications of HCV such as liver decompensation and hepatic cancers.
Chief Medical Editor: Dr. Léna Coïc, Montréal, Quebec
SVR Achieved in 12 Weeks
In pivotal HCV genotype 1 (G1) trials, SVR rates climbed dramatically in both untreated patients and previous pegylated interferon (pegIFN) and ribavirin (R) failures when triple therapy (PI/pegIFN/R) was compared to pegIFN/R alone. Two PIs, telaprevir and boceprevir, are now approved in Canada for HCV G1, but the relative advantages of PI therapy are poised to improve on the basis of new data with simplified regimens.
At EASL, the interim analysis from CONCISE, a phase IIIb study of telaprevir delivered over 12 weeks in patients with IL28B CC genotype suggests undiminished SVR rates even in twice-daily (b.i.d.) dosing. “We can improve PI therapy with less frequent dosing, by reducing treatment duration, as well as by expanding therapy to special populations,” observed Dr. Maria Buti, Liver Unit, Hospital Universitari Vall d’Hebron, Barcelona, Spain. “The excellent 12-week results we are seeing here with telaprevir have important implications for moving toward even better outcomes for this disease.”
In the interim results from the CONCISE trial, extended rapid virologic response (eRVR), signifying undetected HCV at 4 and 12 weeks, was achieved in 99% of patients randomized to 12 weeks of telaprevir/pegIFN/R therapy and 98% of those randomized to 12 weeks of telaprevir with 24 weeks of pegIFN/R. In past studies, eRVR has been highly predictive of overall SVR. The high rates of eRVR were observed in those with prior relapse after HCV treatment as well as to those treatment-naive.
Importantly, the shortened regimen employed telaprevir in a b.i.d. dosing which was found in a previously reported phase III trial, called OPTIMIZE, to provide efficacy commensurate with a q8-hour regimen. “These interim results suggest the potential for treating a selected patient population with the triple therapy for only 12 weeks,” reported senior author Dr. David R. Nelson, Dean of Clinical Research, University of Florida, Gainesville. He suggested the findings in this population of IL28B CC genotype patients have broad implications for cost and convenience but also for outcome in the real-world setting when adherence is an issue.
In this study, 159 of the 239 patients who completed 12 weeks of treatment had a rapid virologic response (RVR) at week 4, defined as HCV RNA <25 IU/mL. These were randomized in a 2:1 ratio to complete treatment at 12 weeks or to remain on therapy for an additional 12 weeks. In the interim analysis, data were available on 115 patients. The safety profile of the 12-week regimen with telaprevir was similar to that observed in previously reported multicentre studies.
OPTIMIZE Data
New data from one of those studies, OPTIMIZE, was still being generated at the most recent EASL where the efficacy and safety of b.i.d. relative to t.i.d. dosing was evaluated in specific populations. OPTIMIZE was the first phase III study of b.i.d. telaprevir. The main results, which found 1125 mg b.i.d. telaprevir to be non-inferior to 750 mg t.i.d. telaprevir, when combined with pegIFN/R in HCV G1 patients, were initially presented as a late-breaker at the 2012 meeting of the American Association for the Study of Liver Diseases.
Figure 1. OPTIMIZE: SVR at 12 Weeks by IL28B Genotype and Fibrosis Stage
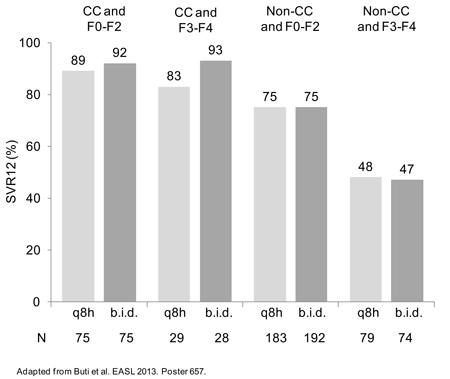
In one subgroup stratification presented at the EASL by Dr. Buti, the efficacy of b.i.d. was similar to t.i.d. even irrespective of IL28B CC genotype (Figure 1). “When outcomes were considered in the context of IL28B genotype or fibrosis state, we found RVR and the subsequent SVR rates to be higher in those with IL28B genotype, relative to those without, and those with lower relative to higher fibrosis stages, but the efficacy of b.i.d. vs. the t.i.d. regimens by these stratifications was similar,” Dr. Buti reported.
In the OPTIMIZE analysis of the impact of anemia on results, Dr. Stefan Zeuzem, Goethe University Medical Center, Frankfurt am Main, Germany, reported that anemia was more common in older patients, patients with cirrhosis and patients who started with a lower hemoglobin count, but it could not be related to b.i.d. vs. t.i.d. telaprevir. While reducing the dose of ribavirin was effective in reducing treatment-related anemia, it did not adversely affect rates of SVR achieved with either of the telaprevir regimens.
Finally, an OPTIMIZE substudy was able to link adherence with treatment success. In data presented by Dr. William Sievert, Monash Medical Centre, Melbourne, Australia, mean telaprevir adherence rates reported using the e-diary were significantly higher with telaprevir BID compared with an every-eight-hour dosing regimen by both imputed and observed data sets (P<0.05 for each). In a multivariate analysis, higher telaprevir adherence almost doubled the odds ratio (OR) of achieving SVR 12 weeks after stopping treatment (OR 1.86; 95% CI, 1.29-2.69; P=0.0008).
HIV Coinfection
Phase III data with b.i.d. boceprevir in HCV are not available, but small studies suggesting activity of b.i.d. boceprevir have been conducted in patients
co-infected with HCV and HIV. However, PIs may not be interchangeable based on an analysis of consecutive patients treated in four clinics that pooled their data.
In a report on 274 HCV patients which included those co-infected with HIV, the RVR was lower on boceprevir plus pegIFN/R than telaprevir plus pegIFN/R (60% vs. 84%; P<0.0001) (Figure 2).
Figure 2. Rapid Virological Response at Week 4: All Patients
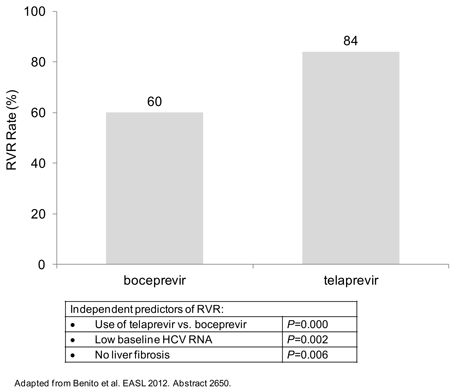
While a low baseline HCV RNA (P=0.002) and an absence of advanced liver fibrosis (P=0.006) were independent predictors of RVR, use of telaprevir was the strongest predictor in a multivariate logistic regression analysis.
“Although we did not find that HIV co-infection influences RVR, the data in this real-world setting suggest that telaprevir may be the best option in more difficult-to-treat patients, such as those with a high baseline HCV RNA or advanced liver fibrosis,” reported Dr. Vicente Soriano, Hospital Carlos III, Madrid, Spain. Although he cautioned that no head-to-head comparison has yet been conducted between PIs in HCV, these data were collected at a time that both PIs were being offered in t.i.d. regimens.
A Vision for the Future:
pegIFN/R-free Regimens
Other PIs may also be soon introduced, including simeprevir, for which phase III data were presented at EASL, and asunaprevir, which has entered phase IIb studies. While the phase III trials with simeprevir, called QUEST1 and QUEST2, were promising, both were limited to treatment-naive patients. A phase III trial in treatment-experienced patients is ongoing, but all studies involve at least 24-week regimens. In its development, asunaprevir is one of several drugs being evaluated in an all oral combination therapy that avoid pegIFN/R by substituting experimental agents, such as daclatasvir, a non-nucleoside inhibitor of NS5B polymerase. Such combinations may be a critical development for those intolerant to pegIFN/R.
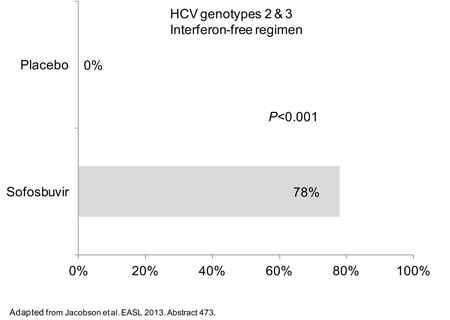
Clinical data with several other agents potentially suitable for an all-oral, pegIFN/R-free combination were also described at the EASL. This included two phase III studies with sofosbuvir, an oral nucleotide inhibitor of NS5B polymerase. Presented by Dr. Ira M. Jacobson, Chief, Division of Gastroenterology and Hepatology, Weill Medical School, Cornell University, New York City, New York, and simultaneously published on line by the New England Journal of Medicine, both studies enrolled patients with genotypes 2 and 3. In one, patients for whom pegIFN was not an option were randomized to sofosbuvir or placebo. The SVR rate was 78% on the active combination vs. 0% in the placebo group (P<0.001) (Figure 3). In the other, patients who had not responded previously to pegIFN were randomized to 12 weeks of sofosbuvir and ribavirin followed by 4 weeks of placebo or 16 weeks of sofosbuvir and ribavirin. SVR rates at 16 weeks were 50% and 73% respectively (P<0.001). Speaking of the first study, Dr. Jacobson, who called these results “highly encouraging,” noted that “there are currently no therapies for HCV patients with a contraindication for interferon.”
Figure 3. POSITRON: SVR at 12 Weeks
Development of other alternative agents is also advancing. For example, phase I results were presented with ALS-2200, which, like sofosbuvir, is a once-daily NS5B polymerase nucleotide inhibitor. In the double-blind randomized study, the median decrease from baseline in HCV RNA viral load at 7 days in HCV G1 patients with compensated cirrhosis was 4.19 log10. There were no serious adverse events.
“There is a need for medications with new mechanisms of action in HCV that are safe and well tolerated and may allow us to avoid IFN and ribavirin,” explained the senior author, Dr. Patrick Marcellin, Head, Viral Hepatitis Research Unit, Hôpital Beaujon, Clichy, France. “ALS-2200 at 200 [mg/day] for 7 days was well tolerated and showed strong antiviral activity in treatment-naive patients with compensated cirrhosis.”
Strategies of combining agents like ALS-2200 and daclatasvir to treat HCV without IFN or ribavirin are being considered as part of the rapid evolution to increase SVR in broader groups of patients. According to Dr. Graham R. Foster, Queen Mary University, London, UK, the current progress has already established new standards for the large proportion of patients inadequately responsive to the standard pegIFN/R regimens. With the evolution, he acknowledged that the challenge for many physicians is to decide whether to move forward with current options or wait for something even better in the most challenging cases, but he argued for moving to triple therapy in most challenging cases because of the advantages that include an improvement in quality of life, a reduced risk of HCV transmission and protection against progression of fibrosis, which can advance unpredictably.
“The next generation agents do look like they are going to have some advantages, such as more convenience and less duration, but I do not think these will show a significant improvement in efficacy relative to the PIs we now have available,” Dr. Foster suggested. Although he believes that regimens that eliminate IFN and ribavirin may provide a significant advance for a large number of HCV patients, he believes that the best approach “is to act now with standard of triple therapy” in order to avoid the rising rates of serious adverse events and diminishing likelihood of SVR as HCV advances.
Summary
The introduction of PI therapy for the treatment of HCV has proven to be a major evolution in the control of this disease. SVR in the most difficult cases can be several times higher than achieved with pegIFN/R alone. However, this evolution is ongoing. At the EASL, new data outline several potential advances, including simplified regimens with existing PI therapies and the introduction of drugs which, when combined with PIs or other agents, may permit IFN and/or ribavirin to be eliminated. The data overall promise major changes in the routine care of HCV.