Reports
Therapies for Indolent Lymphomas: Progress in Efficacy and Tolerability
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
PHYSICIAN PERSPECTIVE - Viewpoint based on literature: Rummel et al. Lancet 2013;381(9873):1203-10.
May 2013
Reviewed and Edited by:
Laurie H. Sehn, MD, MPH
Clinical Associate Professor
BC Cancer Agency and
The University of British Columbia
Vancouver, British Columbia
Introduction
Consensus about the optimal first-line approach to indolent lymphomas, a subset of non-Hodgkin lymphomas (NHLs), is strengthening on the basis of trial evidence. While the value of any individual treatment over another for indolent lymphoma was once unclear, survival benefits in several indolent subtypes, such as symptomatic follicular lymphoma, have now been established in randomized trials. In the most recent phase III comparison, bendamustine plus rituximab was compared to cyclophosphamide, doxorubicin, vincristine and prednisone plus rituximab (CHOP-R), a widely used combination. Bendamustine and rituximab produced a significantly longer progression-free survival (PFS) and was better tolerated. The improved tolerability is important in the context of indolent behaving lymphomas, in which attention to sustaining an optimal quality of life is crucial in view of the prolonged survival. Although there may never be a single standard of care for indolent lymphoma due to the heterogeneity of these malignancies, the goals of therapy are now more clearly defined.
Background
Indolent lymphoma is a term employed to categorize a heterogeneous subset of NHLs characterized by a chronic relapsing-remitting course during which prolonged survival is common. Due to the slow growth of indolent NHL (iNHL), a watch-and-wait approach was once widely used even in symptomatic disease.1 This approach, which is still commonplace in the management of asymptomatic iNHL, was driven by concern for imposing toxicity with modest or negligible change in the clinical course. The benefits of intervening in symptomatic disease are now well accepted on the basis of a series of randomized clinical studies with rituximab, a CD20 monoclonal antibody, which has repeatedly demonstrated strong activity with good tolerability when used alone or in combination with chemotherapy.1-7 Based on this activity, including a survival benefit demonstrated when it was added to cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP-R),3 several major guidelines have endorsed rituximab-containing chemotherapy regimens in advanced iNHL.8,9
Indolent lymphomas, also known as low grade lymphomas, represent about 40% to 50% of the approximately 7800 cases of NHL diagnosed each year in Canada.10 Due to the descriptive nature of the terminology, the World Health Organization (WHO) abandoned the term indolent lymphomas in favour of classification by cell of origin and pathophysiology.11 The most common indolent histology is follicular lymphoma (FL), which represents about 20% of all lymphomas and is derived from germinal B cells.12
Of the 30 types of NHL that have been described, most of which are of B-cell rather than T-cell origin, some remain difficult to classify as aggressive or indolent. Mantle cell lymphoma (MCL), for example, which represents about 3% to 10% of NHL, has a relatively poor prognosis but can behave indolently in some patients, and has occasionally been included in indolent lymphoma treatment trials.13
Evolving Care of Indolent Lymphoma
In the 1980s, a watch-and-wait approach was supported by a series of studies unable to demonstrate any change in the survival trajectory of low-grade NHL when therapy was initiated early relative to late.14,15 The subsequent incremental improvement in the activity of therapeutic agents, particularly with the introduction of rituximab, has provided a rationale for treatment of symptomatic iNHL patients.16
In the trials supporting active treatment, particular emphasis has been placed on demonstrating improvement in clinically relevant outcomes such as time to treatment failure (TTF) or progression-free survival (PFS) in the context of acceptable tolerability to ensure a favourable benefit-to-risk ratio.
Several large studies have provided support for the combination of rituximab and chemotherapy in symptomatic iNHL. In a phase III randomized study, cyclophosphamide, vincristine and prednisone plus rituximab (CVP-R) was compared to CVP alone in 321 patients with previously untreated stage 3 or 4 FL.7 Eight cycles of treatment were delivered in each arm. On the primary end point of TTF, the advantage of CVP-R was highly significant (P<0.0001). In addition, CVP-R also improved both time to progression (P<0.0001) and overall survival (OS) at 4 years (83% vs. 77%; P=0.029). The relative advantage of rituximab was present regardless of bulk of disease or risk group as defined by the Follicular Lymphoma International Prognostic Index (FLIPI). Again, the addition of rituximab was well tolerated although myelosuppression was more common in the CVP-R arm.
A subsequent phase III comparison of CHOP-R to CHOP randomized 428 patients with untreated, advanced stage FL.3 Relative to CHOP, CHOP-R was associated with a higher overall response rate (P=0.011) as well as a significantly prolonged TTF (P<0.001) and prolonged duration of remission (P=0.001). A modest difference in OS favouring CHOP-R also reached significance (P=0.016). Relative to CHOP alone, the addition of rituximab was well tolerated with the difference in adverse effects largely confined to infusion-related reactions and myelosuppression.
As a result of these trials, CHOP-R and CVP-R became widely used. Although the relative efficacy of these treatments was not initially tested, a recently reported 3-arm study demonstrated that both CHOP-R, as well as fludarabine and mitoxantrone combined with rituximab (R-FM) may be more effective than CVP-R.17
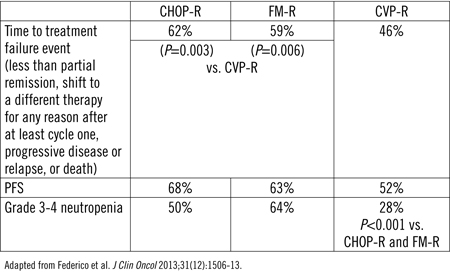
In this study, 534 patients with advanced FL were randomized. After a median of 34 months of follow-up, CHOP-R (P=0.003) and FM-R (P=0.006) were associated with a significantly greater TTF than CVP-R (Table 1). The PFS rates were also significantly higher on CHOP-R and R-FM relative to CVP-R, but OS rates were similar. All three regimens were well tolerated, but R-FM was associated with higher toxicity overall, particularly significantly greater grade ≥3 neutropenia, as well as a higher rate of secondary cancers.
Table 1. FOLLO5 Trial Results at Median Follow-up of 34 Months
Phase III Focus on Benefit-to-Risk Ratio
Despite the favourable effects on clinically relevant outcomes, the toxicity of most standard chemotherapy regimens has remained a concern within the goal of preserving quality of life over a prolonged period of survival. Of alternative drugs to combine with rituximab, the cytotoxic alkylating agent bendamustine has attracted attention because of persistent activity in patients refractory to other therapies and its low relative risk of the types of adverse events that adversely affect quality of life, such as alopecia and peripheral neuropathy.18,19 Bendamustine was first approved in the US in 2008 but has been in clinical use for more than 2 decades in Germany, where it is approved for treatment of rituximab-refractory lymphoma.20
In a recently completed phase III trial designed to compare rituximab plus bendamustine (B-R) to CHOP-R in iNHL and mantle cell lymphoma, the hypothesis was that B-R would be non-inferior to CHOP-R but better tolerated.21 It was initiated in 2003 at 81 participating study centres in Germany by the STiL study group. PFS was the primary end point. Secondary end points included overall response (ORR), complete response (CR), time to next antilymphoma treatment and toxicity. Key entry criteria included a stage III or IV indolent lymphoma or MCL and a WHO performance status of ≤2. The iNHL subtypes included FL, lymphoplasmacytic (Waldenström’s macroglobulinemia), small lymphocytic and marginal-zone lymphoma.
In an open-label format, 274 patients were randomized to B-R and 275 to CHOP-R. In both groups rituximab 375 mg/m2 was administered on day 1 of each cycle. On the every 4-week 2-drug regimen, bendamustine 90 mg/m2 was administered on days 1 and 2. On the every 3-week CHOP regimen, cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2 and vincristine 1.4 mg/m2 were administered on day 1 while prednisone 100 mg was administered on days 1 to 5. Both groups were treated for a maximum of 6 cycles. No maintenance regimen was administered
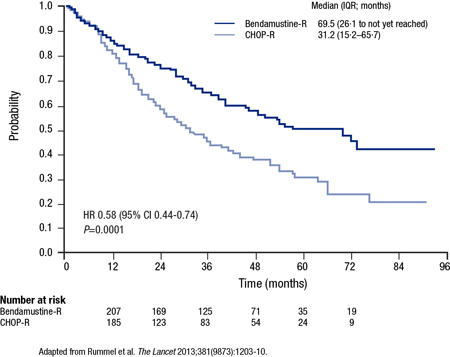
After a median follow-up of 45 months, the median PFS was 69.5 months on B-R vs. 31.2 months on CHOP-R, yielding a 42% hazard ratio (HR) reduction (HR 0.58; 95% CI, 0.44-0.75; P<0.0001) (Figure 1). The relative advantage of B-R was independent of age, lactate dehydrogenase level and FLIPI score. B-R was also favoured for many of the secondary end points, including CR (40% vs. 30%; P=0.021) and time to next antilymphoma treatment (not reached vs. 42.3 months; P<0.0001). However, OS was not different between the cohorts (P=N/S).
Figure 1. StiL PFS Results
Adverse events were less common on B-R. In those who received at least 3 cycles of therapy, this included significantly lower rates of alopecia (0% vs. 100%), peripheral neuropathy (7% vs. 29%; P<0.0001) and stomatitis (6% vs. 19%; P<0.0001). Erythematous skin reactions, which occurred more often on B-R (16% vs. 9%; P=0.024), was an exception. Overall, neurotoxic effects were 4 times more common on CHOP-R than on B-R.
Most hematological toxic effects were also significantly less common on B-R relative to CHOP-R. This included grade 3 or 4 leukopenia (P<0.0001) and grade 3 or 4 neutropenia (P<0.0001). However, patients receiving B-R experienced more lymphopenia (grade ≥3, 74% vs. 43%; P value not disclosed). Infectious episodes of any grade were more common on CHOP-R than B-R (50% vs. 37%; P=0.0025). Sepsis was uncommon in both groups but occurred significantly more frequently on CHOP-R (3% vs. <1%; P=0.019). Patients receiving B-R were less likely to be given hematopoietic growth factors. Secondary malignancies were identified in 20 B-R patients vs. 23 CHOP-R patients.
Of the 4 primary histological subgroups represented in this study (FL, MCL, Waldenström’s macroglobulinemia, and marginal zone lymphoma), the PFS advantage of B-R relative to CHOP-R was statistically significant for all but marginal zone lymphoma. Expressed in terms of HR, these were 0.61 for FL (P=0.0044), 0.49 for MCL (P=0.0044), 0.33 for Waldenström’s macroglobulinemia (P=0.0033) and 0.7 for marginal-zone lymphoma (P=0.3279). The favourable direction of benefit may not have reached significance in this last group due to small numbers.
Based on previously reported study results22, several updated guidelines, including those of the National Comprehensive Cancer Network (NCCN) and the European Society of Medical Oncology (ESMO), have already identified B-R as a first-line treatment option in indolent lymphomas. In Canada, the results of this study were influential in producing a positive pan-Canadian Oncology Drug Review (pCODR) decision and several provincial recommendations for B-R in the first-line treatment of iNHL and MCL.
Conclusion
With an increasing number of effective treatment options, the primary goal in indolent lymphoma is now focused on achieving durable control of disease, while minimizing toxic side effects. The benefit:risk ratio of active front-line therapies remains an important concern, with aim to maximize quality of life. Relative to CHOP-R, which has achieved high rates of activity when compared to other rituximab-based combinations, the more effective and better tolerated B-R regimen may be emerging as a preferred alternative. The next advances in the control of indolent lymphomas will continue to focus on strategies that improve activity, while minimizing adverse effects.
References
1. Lunning MA, Vose JM. Management of indolent lymphoma: where are we now and where are we going. Blood Rev 2012;26(6):279-88.
2. Lowry L, Ardeshna KM. Has single-agent rituximab replaced watch-and-wait for a patient with asymptomatic low-grade follicular lymphoma? Cancer J 2012;18(5):390-5.
3. Hiddemann et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 2005;106(12):3725-32.
4. Herold et al. Rituximab added to first-line mitoxantrone, chlorambucil, and prednisolone chemotherapy followed by interferon maintenance prolongs survival in patients with advanced follicular lymphoma: an East German Study Group Hematology and Oncology Study. J Clin Oncol 2007;25:1986-92.
5. Marcus et al. CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood 2005;105:1417-23.
6. Salles et al. Rituximab combined with chemotherapy and interferon in follicular lymphoma patients: results of the GELA-GOELAMS FL2000 study. Blood 2008;112:4824-31.
7. Marcus et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol 2008;26(28):4579-86.
8. Zelenetz et al. NCCN Clinical Practice Guidelines in Oncology: non-Hodgkin’s lymphomas. Version 1.2013. 2013: Available at: www.nccn.org.
9. Ghielmini et al. ESMO Guidelines consensus conference on malignant lymphoma 2011 part 1: diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), and chronic lymphocytic leukemia (CLL). Ann Oncol 2013;24:561-76.
10. NHL Statistics. 2012. (Accessed March 7, 2013, at http://www.nhlcyberfamily.org/statistics.htm#canada.)
11. Jaffe et al. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press 2001.
12. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood 1997;89(11):3909-18.
13. Hoster et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood 2008;
111(2):558-65.
14. Horning SJ, Rosenberg SA. The natural history of initially untreated low-grade non-Hodgkin’s lymphomas. N Engl J Med 1984;311(23):1471-5.
15. Young et al. The treatment of indolent lymphomas: watchful waiting v aggressive combined modality treatment. Semin Hematol 1988;25(2 Suppl 2):11-6.
16. McLaughlin P. Progress and promise in the treatment of indolent lymphomas. Oncologist 2002;7(3):217-25.
17. Federico et al. R-CVP versus R-CHOP versus R-FM for the initial treatment of patients with advanced-stage follicular lymphoma: results of FOLLO5 trial conducted by the Fondazione Italiana Linfomi. J Clin Oncol 2013; 2013;31(12):1506-13..
18. Cheson BD, Rummel MJ. Bendamustine: rebirth of an old drug. J Clin Oncol 2009;27(9):1492-501.
19. Rummel MJ, Gregory SA. Bendamustine’s emerging role in the management of lymphoid malignancies. Semin Hematol 2011;48 Suppl 1:S24-36.
20. Kahl et al. Bendamustine is effective therapy in patients with rituximab-refractory, indolent B-cell non-Hodgkin lymphoma: results from a Multicenter Study. Cancer 2010;116(1):106-14.
21. Rummel et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 2013;381(9873):1203-10.
22. Rummel et al. Bendamustine plus rituximab (B-R) versus CHOP plus rituximab (CHOP-R) as first-line treatment in patients with indolent and mantle cell lymphomas (MCL): Updated results from the StiL NHL1 study. J Clin Oncol 2012;30:(suppl;abstr 3).