Reports
Simplifying the Management of Chronic Obstructive Pulmonary Disease
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
MEDICAL FRONTIERS - International Conference of the American Thoracic Society
San Francisco, California / May 18-23, 2012
San Francisco - Bronchodilators are central to the management of symptomatic chronic obstructive pulmonary disease (COPD). Both long-acting beta agonists (LABAs) and anticholinergics (LAACs) have been an important step toward minimizing daily dosage requirements from the standard 4-times-daily to twice-daily administration. While the only LAAC currently available in a once-a-day dosing is tiotropium (also referred to as a long-acting muscarinic antagonist), new once-daily LAACs are under development. The first approved once-daily LABA is now indicated for the treatment of COPD. The weight of clinical evidence supporting once-daily dosing has been presented here at this year’s ATS conference.
Chief Medical Editor: Dr. Léna Coïc, Montréal, Quebec
Chronic obstructive pulmonary disease (COPD) is characterized by progressive airflow limitation which does not fully improve in response to therapy. Currently, it is estimated to affect approximately 10% of the adult population and projections indicate that it could become the second leading cause of death by the year 2020. Of the many symptoms reported by COPD patients, dyspnea is the most bothersome. In a cohort of over 42,000 COPD patients identified in the UK General Practice Research Database, Mullerovah et al. reported here that 44% had clinically significant dyspnea as reflected by a Medical Research Council (MRC) score of ≥3 despite treatment. In this UK COPD primary care cohort, only 18% of patients did not experience dyspnea and shortness of breath as observed across all grades of COPD, including mild or Global Initiative for Obstructive Lung Disease (GOLD) stage I.
Patients who experience acute exacerbations may require admission to the intensive care unit (ICU). As demonstrated by a retrospective study of American patients admitted to an ICU at a tertiary-care academic centre, long-term outcomes following ICU admission are considerably poorer for COPD patients. The median survival following ICU admission was 11 months for COPD patients vs. 27.2 months for non-COPD patients admitted (P<0.001). Of this cohort, at 5 years, only 13% of COPD patients were alive compared to over 37% for the non-COPD patients.
Phenotype and Outcome
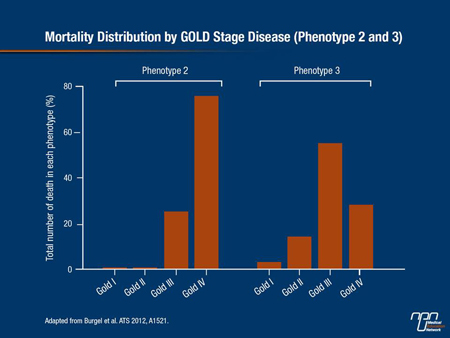
Findings indicate that some patients with COPD are at substantially greater risk for mortality than others. In an effort to identify phenotypes of COPD with differing mortality risks, Burgel et al. analyzed a group of 527 stable COPD patients based on clinical data, CT scans and comorbidities.
phenotype 1 patients and was associated with severity of airflow limitation. Phenotype 3 included older patients approximately 75 years of age, with moderate airflow limitation and less severe emphysema on CT scan but higher BMI and CV comorbidity rates. The adjusted HR for mortality for phenotype 3 patients, although lower than for phenotype 2, was still 14.3 times greater than mortality for phenotype 1 patients (Figure 1).
Figure 1.
Advances in COPD Management:
LABA with Once-daily Dosing
Bronchodilators either in the form of long-acting beta agonists (LABAs) or the long-acting anticholinergics (LAACs) are central to the symptomatic management of COPD. Until recently, formoterol and salmeterol were the only available LABAs for COPD, both of which require twice-daily dosing.
LABAs administered once-daily are a step forward in the management of COPD. Under investigation is the LABA olodaterol which has a confirmed duration of action of at least 24 hours in both preclinical and clinical studies. In a phase II study of 47 COPD patients, 5 µg q.d. demonstrated a better 24-hour profile compared with 2 µg b.i.d. (abstract D60).
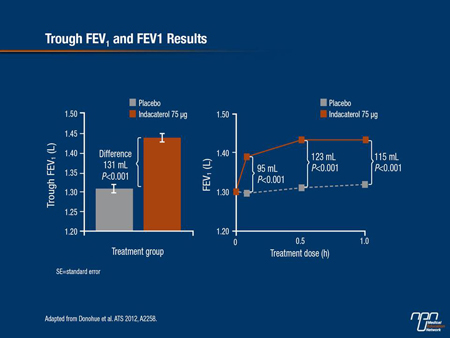
The first approved once-daily dosing LABA for maintenance treatment of COPD is indacaterol. The 75 µg/day dosage provides 24-hour bronchodilation as reflected by sustained trough FEV1 improvements until the next daily dose. In a pooled analysis of 2 identically designed phase III studies, Donohue et al. reported that indacaterol 75 µg significantly increased trough FEV1 at week 12 relative to placebo (P<0.001), with a mean difference of 131 mL between the 2 arms. Investigators also found that FEV1 improved within 5 minutes of the first dose, leading to an increase of 95 mL within the first hour post-dose on day 1 (P<0.001 vs. placebo) (Figure 2).
Figure 2.
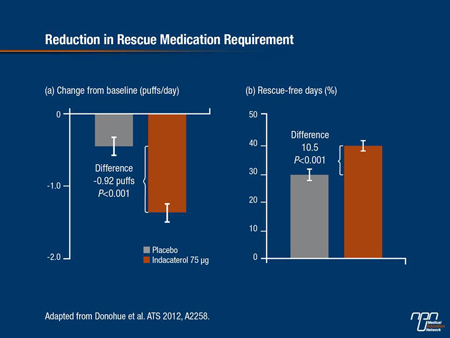
It also reduced the need for rescue albuterol over the 12 weeks of treatment with 10.5 rescue free-days compared with placebo (P<0.001) (Figure 3).
Improvements in both dyspnea and the SGRQ were also seen with the 75 µg dose vs. placebo. As noted by Mahler et al., improvements from baseline in the minimally clinically important difference (MCID) in St. George’s Respiratory Questionnaire (SGRQ) total scores of between 4 and 8 units indicate that treatment has been either effective or very effective. At week 12, health status as reflected by changes in the SGRQ total score significantly improved with the once-daily LABA vs. placebo with a mean difference of -3.8 units (P<0.001).
At week 12, the Transition Dyspnea Index (TDI) total score was also significantly improved with active therapy vs. placebo, with a mean difference of 0.8 between the 2 arms (P<0.001). In fact, 47.7% of patients in the indacaterol arm achieved MCID in TDI total score by week 12 vs. 33.8% of placebo controls (P<0.001), while 49.2% of them achieved a MCID in the SGRQ total score compared with 35.9% of placebo controls (P=0.001). Overall frequency of adverse events (AEs) was similar between treatment groups and numerically more serious AEs occurred in placebo patients than those treated with the once-daily LABA.
Figure 3.
Under Development
To date, once-daily tiotropium is the only LAAC available for the treatment of COPD (also referred to as a LAMA) and is a mainstay in respiratory disease management. However, other LAACs are currently under investigation and may broaden the class in future COPD management. Aclidinium bromide is in the advanced stages of development. A phase III study in 600 COPD patients confirmed its long-term efficacy and safety when given twice-daily. Additionally, phase III results were presented for glycopyrronium bromide NVA237, another LAAC not yet commercially available.
Analyses from the GLOW2 studies comparing NVA237 to placebo and to open-label tiotropium show it offers clinically meaningful benefits in moderate-to-severe COPD. In an analysis presented here at the ATS by Kerwin et al., the mean trough FEV1 was superior at week 12 for both NVA237 (97 mL) and for open-label tiotropium (83 mL) compared with placebo (P<0.001). The investigational agent also provided rapid bronchodilation following the first dose on day 1, leading to significantly higher FEV1 levels from 5 minutes to 2 hours post-dose vs. tiotropium (P<0.01)
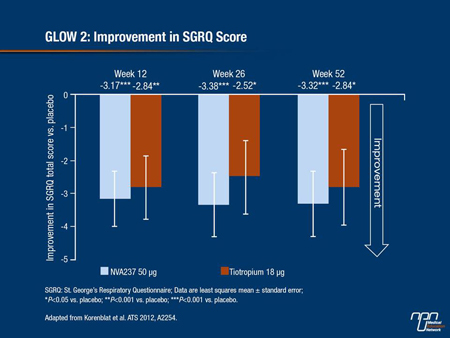
Korenblat et al. also found that NVA237 significantly improved the TDI at week 26 (2.13 vs. 1.32 for placebo)—a level of improvement comparable to that seen with tiotropium. The percentage of patients who achieved an MCID ≥1 point in the TDI score was also significantly higher with the new molecule at 55.3% vs. 44.2% for placebo and was again comparable to that achieved with open-label tiotropium. Changes in SGRQ score at week 52 also significantly favoured the new molecule compared with placebo (P<0.001) and were comparable to improvements seen with tiotropium.
Figure 4.
Further analyses of the same GLOW2 data set showed that NVA237 significantly prolonged time to the first moderate exacerbation by 34% vs. placebo over 52 weeks (P<0.001), with a number-needed-to-treat of 13.2 to prevent 1 exacerbation. The investigational agent also reduced moderate to severe exacerbations by 35% over placebo (P=0.003). In contrast, the effect of tiotropium on this particular end point was not statistically significant compared with placebo.
The overall AE rate was similar across all 3 treatment groups while serious AEs were slightly less frequent in the NVA237 group compared with placebo and open-label tiotropium.
Summary
Patients with COPD frequently experience acute exacerbations that require admission to the ICU. Non-COPD patients admitted to an ICU have a survival rate almost threefold greater than COPD patients; phenotyping can better identify those individuals at greater risk. The reduction in daily dosing requirements of LABAs from the average 4 times a day to twice-daily dosing has helped simplify disease management. As the first LABA to be approved as a once-daily treatment for COPD, indacaterol has demonstrated rapid and sustained action and may enhance patient adherence. Along with novel LAACs in development, treatment algorithms continue to undergo clinically important changes.
Questions and Answers
Dr. Reynold Panettieri, Professor of Medicine, University of Pennsylvania; Dr. Donald Mahler, Professor of Medicine, Dartmouth Medical Center, Hanover, New Hampshire; and Dr. James Donohue, Professor of Medicine, University of North Carolina School of Medicine, Chapel Hill.
Q: Why is it important to achieve rapid bronchodilation in COPD?
Dr. Panettieri: The speed at which patients experience improvement in FEV1 is important because I have to get my patients to use their drugs as prescribed; if I get a demonstrable change in critical outcomes like the FEV1, that may translate into patients using their drug more regularly. So patients who use a controller medication from which they get quick relief are more likely to sustain its use.
Q: What advantages do you think a once-daily inhaler may offer over twice-daily dosing?
Dr. Mahler: We used to have medicines that lasted 4 hours like albuterol and then along came tiotropium and it was shown to be more effective and led to better compliance than 4-times-a day albuterol. So clearly, longer acting was better for the muscarinic antagonists. For the beta agonists, we have formoterol and salmeterol, both of which were shown to be better than 4-times-a-day albuterol as well. So our current paradigm suggests that longer acting is more efficacious and compliance is better than with more frequent dosing. If you extrapolate that, I would argue that once a day is probably better than twice a day until we have evidence to the contrary. And patients can always use rescue albuterol if they need it.
Q: In Europe, indacaterol was approved at doses of 150 µg and 300 µg, while in Canada and the US, the approved dose for COPD maintenance therapy is 75 µg. Should physicians be tempted to double the dose to get patients up to approved European doses?
Dr. Donohue: Not at all, nor should they add a second dose—I strongly recommend they don’t do that. If patients are doing well on 75 µg, leave it alone and let the FDA tell us that we can go to 150 µg a day or not. In our European studies, the effect size on lung function was a bit larger with the 150-µg than with the 75-µg dose, but the differences between the 2 doses are probably not clinically meaningful, and declines in the SGRQ were actually larger for the 75-µg dose than with the 150-µg dose. So 75 µg is a highly effective dose.