Reports
Updates on aHUS: Latest Insights into Triggers, Diagnosis, and Management Outcomes
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
PRIORITY PRESS - Kidney Week 2022 – American Society of Nephrology (ASN)
In-person/virtual, Orlando, Florida / November 3–6, 2022
In-person/Virtual – Atypical hemolytic uremic syndrome (aHUS) is a form of thrombotic microangiopathy (TMA) that primarily affects the kidney but has a wide variability in its triggers and clinical presentation. In spite of its ultra-rare status, aHUS was a topic of interest at Kidney Week, the flagship conference of the American Society of Nephrology. Nineteen posters and four publication-only abstracts covered various facets of aHUS, using case studies and larger-scale analyses from clinical trials and the Global aHUS Registry to deepen our understanding of aHUS triggers and diagnosis, and to give us new perspectives on the long-term clinical outcomes with complement C5 inhibitor medications.
Chief Medical Editor: Dr. Léna Coïc, Montréal, Quebec
“aHUS is caused by inherited or acquired abnormalities in the alternative pathway of complement. It includes the classic triad of microangiopathic hemolytic anemia, thrombocytopenia, and acute kidney injury, though other organs can also be affected,” explained Dr. Larry Greenbaum, Emory University School of Medicine in Atlanta, Georgia, in his poster presentation. “Prior to the development of the C5 inhibitor eculizumab, aHUS outcomes were poor; end-stage kidney disease, morbidity and mortality were common.”1
In an interview, Dr. Christoph Licht, SickKids Hospital in Toronto, Ontario, gave more details about our current understanding of aHUS development. “Complement-mediated diseases generally need more than one “hit” to develop – you have a susceptibility to manifesting this disease, whether it’s a genetic mutation or the presence of antibodies, or something else that we aren’t able to pinpoint yet,” he said. “Then, there’s an inflammatory or infectious event that adds to the complement system’s activity, and that leads to disease.”
Focus on aHUS Triggers
To investigate some of these second “hits” that may trigger aHUS, Dr. Andrew Siedlecki, Brigham and Women’s Hospital in Boston, Massachussetts, and colleagues analyzed potential triggering events in approximately 1900 patients included in the Global aHUS Registry. In total, 349 individuals experienced a medical event that could be considered an aHUS trigger; such events included malignancy, acute infection, autoimmune disease, kidney transplant, malignant hypertension, neonatal onset, chronic infection, drug treatments, bone marrow transplant, and C3 glomerulopathy. For adults, the most common trigger was malignancy (22%); in children, it was acute infection (36%). The timing of onset depended on the trigger; aHUS triggered by acute infections, malignant hypertension, or kidney transplant generally occurred within a few days to a week, while for chronic infections, drug-induced aHUS, and autoimmune disease, there could be delays of two to three years between the trigger and clinically evident aHUS.2
Dr. Licht, a contributor to the Registry and former chair of its scientific advisory board, provided additional advice based on these findings. “With the different triggers, it’s important to understand the risk windows so you can translate the learnings into action items.” he said. “Infections are generally transient, short-term phenomena so the scenario is simpler, but if malignancy is the trigger, that won’t go away after three weeks or so. In those cases it’s important to provide education to the patient – or the parents of a pediatric patient – about being vigilant for possible disease recurrence if you discontinue the drug.”
Diagnostic Challenges and Potential Tools in aHUS
“Although aHUS is a rare form of TMA, we need to consider it a medical emergency, and consequently early diagnosis is imperative,” said Dr. Miguel Uriol Rivera, Son Espases University Hospital in Palma de Mallorca, Spain, in an email interview. He pointed out that distinguishing aHUS from other types of TMA can be challenging due to the complexity of aHUS pathophysiology and long delays in receiving genetic testing results. One important element of the differential diagnosis is distinguishing aHUS from other forms of TMA, particularly thrombotic thrombocytopenic purpura (TTP). The PLASMIC score was previously developed and validated as a diagnostic tool for TTP; a score of 6 or higher on the seven-point scale is indicative of likely TTP.3 In their poster at Kidney Week, Dr. Uriol Rivera and colleagues investigated whether it could also be helpful for aHUS diagnosis, by analyzing data from patients with confirmed aHUS in trials of the C5 inhibitors eculizumab and ravulizumab. “In our study, 87% of patients presented a PLASMIC score lower than 6, associated with platelet counts higher than 30,000/μL and slight deterioration of renal function (plasma creatinine > 2 mg/dL),” said Dr. Uriol Rivera (Figure 1). “Observing these characteristics when the PLASMIC score is used in patients with a high clinical suspicion of aHUS could facilitate a rapid diagnostic approach in clinical practice.”4
Figure 1.
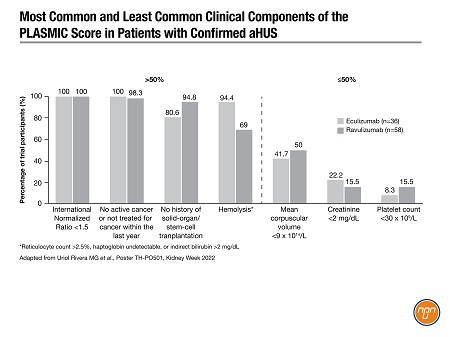
Dr. Uriol Rivera said that the next step will be to validate the PLASMIC score in larger TMA datasets and prospective studies. “Additionally, if the PLASMIC score represents a prognostic approach that facilitates the choice of the best treatment, it would represent an invaluable tool not only for the benefit of patients but also for the sustainability of the health system,” he concluded.
Treatment Outcomes in aHUS
Since its introduction in 2011, the C5 inhibitor eculizumab has become the standard of care for aHUS in Canada, with a profound impact on morbidity and mortality. Just prior to Kidney Week, ravulizumab, an eculizumab derivative with an extended dosing interval, was approved in Canada as a second targeted option for management of aHUS, in adults and in children aged one month and older. Several analyses at Kidney Week investigated the long-term outcomes and best practices for anti-complement therapy in aHUS.
A poster by Dr. Greenbaum and colleagues investigated long-term outcomes in patients in the Global aHUS Registry who received eculizumab for at least 90 days. Cohort A (n=249) did not develop end-stage renal disease (ESRD) over three years of follow-up; patients in Cohort B (n=56) progressed to ESRD after starting eculizumab. Both cohorts showed marked improvements in hematologic parameters from treatment initiation until the last follow-up, and Cohort A also showed improvements in estimated glomerular filtration rate (eGFR). Mortality was higher in Cohort B (14.3%) compared to Cohort A (0.8%). Although there was no clear link between patient baseline characteristics and treatment response or progression to ESRD, the overall findings support the long-term efficacy of eculizumab for improving hematologic and renal outcomes in a significant proportion of patients.1
One of the key questions in aHUS treatment is whether eculizumab should be continued indefinitely, or if in some patients it may be appropriate to interrupt treatment after the initial course and monitor for disease recurrence. A poster by Dr. Romy Bouwmeester, Radboud University Medical Centre in the Netherlands and colleagues reported on a four-year prospective observational study (CUREiHUS) in non-transplanted patients with aHUS who were treated with eculizumab for three months then discontinued. Following discontinuation, only 22% (n=4) of patients experienced a clinical relapse, in spite of the majority (81%) possessing a known complement genetic variant or antibodies that would drive aHUS susceptibility. The patients who relapsed were re-treated with eculizumab and did not suffer any long-term sequelae. Treatment interruption yielded combined healthcare cost savings of approximately 70% compared to continuous treatment. The authors concluded that, “It is safe and (cost-) effective to discontinue eculizumab after three months of treatment in patients with aHUS with native kidneys.”5
Conclusions
Now that larger-scale datasets are available from registries and clinical trials, we can confirm and build on what we understand from case reports and clinical experience about aHUS pathophysiology and clinical outcomes. Novel diagnostic tools such as the PLASMIC score may be helpful for prompt diagnosis and treatment initiation. Complement inhibition remains the gold standard for management of aHUS, providing improvements in mortality and in hematologic and renal outcomes over the long term; in appropriate patients a time-limited course may provide cost savings while maintaining clinical effectiveness.
References:
1. Greenbaum LA, et al. Poster TH-PO502 presented at Kidney Week, Nov 3–6, 2022.
2. Siedlecki A, et al. Poster TH-PO500 ibid.
3. Li A, et al. J Thromb Hemost 2018; 16: 164–169.
4. Uriol Rivera MG, et al. Poster TH-PO501 presented at Kidney Week, Nov 3–6, 2022.
5. Bouwmeester RN, et al. Poster TH-PO503 ibid.