Reports
Complement in the Spotlight: New insights into PNH Management from ASH
This report is based on medical evidence presented at sanctioned medical congress, from peer reviewed literature or opinion provided by a qualified healthcare practitioner. The consumption of the information contained within this report is intended for qualified Canadian healthcare practitioners only.
PRIORITY PRESS - 64th Annual Meeting of the American Society of Hematology (ASH)
In-person/virtual, New Orleans, Louisiana / December 10–13, 2022
New Orleans – Paroxysmal nocturnal hemoglobinuria (PNH) is a rare disorder driven by the inappropriate activity of the complement system against red blood cells. In spite of its rarity, PNH was a topic of keen interest at ASH 2022, where one of the Society’s main prizes recognized Dr. John Atkinson and Dr. Peter Hillmen for their respective contributions to our understanding of complement biology and management of complement-related diseases. A further three sponsored sessions, three podium presentations, and 23 posters explored additional facets of PNH pathophysiology and management. This report focuses on insights into our understanding of PNH clinical outcomes and the impact of established and emerging therapies.
Chief Medical Editor: Dr. Léna Coïc, Montréal, Quebec
“PNH is the archetypal complement-dependent disorder,” explained Dr. Peter Hillmen, University of Leeds, UK and Head of Hematology Engagement for Apellis Pharmaceuticals, in his keynote address for the Ernest Beutler Lecture and Prize at ASH 2022. “As a consequence of complement dysregulation, patients with PNH experience continuous intravascular hemolysis and hemoglobinuria, and symptoms which include anemia requiring regular transfusions. About half the patients have thrombosis, and often it’s in unusual sites. Historically, before treatment, about a third of patients died of thrombosis,” he said. The lectures by Dr. Hillmen and Dr. John Atkinson, Washington University, outlined how our evolving knowledge of complement biology has led to the development of the current standard of care for PNH, inhibition of the terminal complement component C5 by eculizumab and its derivative ravulizumab. Dr. Hillmen discussed additional potential therapeutic targets such as the proximal complement components C3 and factors B and D, and highlighted the central importance of complement not just for PNH, but as a potential contributor to a wide variety of autoimmune and/or inflammatory conditions across multiple body systems.1
Further Insights into the Standard of Care
Eculizumab now has more than 20 years of data that can provide long-term insights into PNH outcomes and the impact of treatment. Dr. Richard Kelly, St James’s University Hospital, Leeds, presented a retrospective analysis of all patients with PNH treated with eculizumab or ravulizumab in the UK since May 2002, and showed that delays and challenges in diagnosis were common, with about 17% of patients being referred to a non-hematologist specialist and around one in eight patients being misdiagnosed with an alternate condition. With C5 inhibitor treatment, most patients (72.4%) have been able to remain transfusion-independent over a one-year period and the rate of thrombosis is low (4.5%). Dr. Kelly concluded that, “Our experience of treatment with C5 inhibition with eculizumab and ravulizumab confirms the continued long-term safety and efficacy of these products. Overall survival remains high and complications such as thrombosis, meningococcal infection, and clonal evolution are uncommon.”2
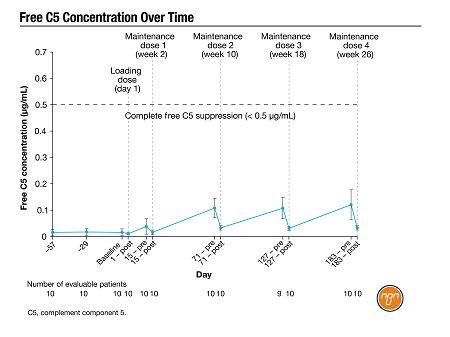
Since ravulizumab is becoming more widely available, many patients previously treated with eculizumab every two weeks are now switching, to take advantage of its longer (eight-week) dosing interval. A poster by Dr. Morag Griffin, St James’s University Hospital, and colleagues presented an interim analysis of a phase 4 trial in 10 patients switching to ravulizumab. Mean lactate dehydrogenase (LDH) did not change significantly following the switch (Figure 1) and free C5 levels were effectively suppressed throughout the dosing interval. No patient experienced breakthrough hemolysis (BTH) during the 183-day analysis period and there were no reports of meningococcal infections or other serious treatment-emergent adverse events (AEs).3
Figure 1.
Emerging Treatment Options
C3 inhibitor: Pegcetacoplan
Pegcetacoplan is a subcutaneously administered C3 inhibitor, recently approved in Canada for treatment of adults with PNH for whom C5 inhibitors are inappropriate or inadequately effective. Dr. Christopher Patriquin, University Health Network, Toronto, Chair of the Canadian PNH Network, presented an extension study across several trials in previously treated and treatment-naïve patients. “The longer-term data are supporting, for the most part, what we saw in the initial [phase 3] PEGASUS study,” he said in an interview. “We saw persistent normalization or close-to-normalization of hemoglobin for patients on therapy, and fairly good LDH control. And that seems to translate into improved quality of life scores and reduced transfusion burden for patients.”4 He added that, as with other complement inhibitors, BTH can occur with pegcetacoplan, and that more data and experience will be needed to determine how best to manage it and how to counsel patients.
Factor B Inhibitor: Iptacopan
In a late-breaking oral session, Dr. Regis Peffault de Latour, French Reference Centre for Aplastic Anemia and Paroxysmal Nocturnal Hemoglobinuria, Paris, presented a randomized phase 3 trial of the oral factor B inhibitor iptacopan versus eculizumab or ravulizumab in PNH patients whose anemia was not adequately managed on a C5 inhibitor. Over 24 weeks of treatment, 51 of the 60 iptacopan-treated patients experienced an increase in hemoglobin from baseline of at least 2 g/dL, and 42 patients achieved an absolute hemoglobin level over 12 g/dL; neither of these endpoints was reached in any patient remaining on a C5 inhibitor. There were no deaths or infections with encapsulated bacteria in either treatment arm; the most common AEs with iptacopan were headache and diarrhea. Iptacopan is not currently available in Canada.5
Factor D inhibitor: Vemircopan
Dr. Peter Browett, University of Auckland, New Zealand, presented the interim results of a phase 2 proof-of-concept study on the oral factor D inhibitor vemircopan in treatment-naïve PNH patients. After the 12-weeks treatment period, patients’ mean increase in hemoglobin from baseline was 3.9 g/dL, and LDH decreased from baseline by 81%. All patients but one remained transfusion-free out to 26 weeks of follow-up. Most AEs were mild to moderate and considered unrelated to the medication, with the most common being headache and vomiting. Dr. Browett concluded that, “This interim efficacy and safety analysis provides proof of concept for factor D inhibition with vemircopan in patients with PNH, and supports the concept of moving to further phase 3 trials.”6 Vemircopan is not yet commercially available in Canada.
Key Takeaways for Canadian Clinicians
“I think the main message stands, not just when deciding between [a C5 inhibitor and pegcetacoplan] but for any upcoming therapy: we have to make sure that terminal complement is blocked,” said Dr. Patriquin. “That's the base - we have to protect our patients from hemolysis and all the untoward effects that can happen there, including thrombosis. And in addition to that, there may be some benefits to being on a proximal inhibitor, particularly for patients who are symptomatically anemic despite being on optimal C5 blockade.”
Dr. Patriquin cautioned that for patients switching to pegcetacoplan from eculizumab or ravulizumab, “We’ll have to monitor them as closely as we would on a C5 inhibitor but possibly more frequently, just because it's a newer area. We need to get a better idea in the clinic of how patients’ disease will behave around things like vaccines and infections - in a global pandemic world, we always have that front of mind.”
He and his colleagues in the Canadian PNH Network also encourage clinicians to call on them as resources and collaborators. “At any point, if you have a PNH patient and that’s new to you, reach out to a PNH expert across the country - we may be able to provide some initial guidance, possibly even enroll them into the registry, and there may even be clinical trials available,” he said. “The more Canadian participation we have in the trials – assuming these therapies prove themselves to be safe and effective – the more likely they'll make it into the commercial market in Canada. So if you find a patient, we're happy to help.”
Conclusions
C5 inhibition with eculizumab or ravulizumab remains the standard of care in PNH, and long-term analyses support the robust efficacy and safety profile of these agents. Switching from eculizumab to ravulizumab can be achieved without compromising disease control. Pegcetacoplan is a new option that may improve outcomes in patients for whom C5 inhibitors are inadequate, but close monitoring and assessment of infection risk are advisable.
References:
1. Atkinson J and Hillmen P. Ernest Beutler Lecture and Prize, presented at ASH, Dec 10–13, 2022.
2. Kell Ry, et al. Poster 2566 at ASH, Dec 10–13, 2022.
3. Griffin M, et al. Poster 1251 at ASH, Dec 10–13, 2022.
4. Patriquin C, et al. Poster 1248 at ASH, Dec 10–13, 2022.
5. Peffault de Latour R, et al. Late-breaking oral presentation LBA-2 at ASH, Dec 10–13, 2022.
6. Browett P, et al. Oral presentation 294 at ASH, Dec 10–13, 2022.